|
HOMEPAGE |
Iridoviruses |
Virus insecticides |
Spinosad |
Mosquitoes blackflies
& ticks |
Predators,
parasitoids, pathogens |
Flies of
agricultural importance |
Students |
Español |
 |
|
Biology and Ecology of Baculoviruses (click here for
publications) |
|
Understanding the ecology of insect
pathogenic baculoviruses requires a basic appreciation of their
biology.
Baculoviruses (comprising nucleopolyhedroviruses and granuloviruses)
have two virion forms.
The occlusion-derived virions infect
insect midgut cells following ingestion of contaminated foliage by
susceptible insect larvae.
These virions fuse with the cell membrane releasing nucleocapsids
into the cell.
The nucleocapsids migrate the nucleus and begin
replication or pass straight through the midgut cell to infect the
cells of other tissues.
Initial replication results in the production of virions that bud
through the basal cell membrane.
These budded virions
disperse throughout the insect.
Later in infection, virions are enveloped (individually or in groups) and
are occluded into large (~1-3 µm) occlusion bodies (OBs) designed
for insect-to-insect transmission.
Multiple enveloping appears to be a strategy for overcoming host
cell responses to viral infection, but this has important
evolutionary consequences because each cell can be infected by
multiple virus genotypes.
Shortly before death, the infected insects become pale and flaccid
and often climb to the apical points of the plant where they die.
The body ruptures releasing millions of occlusion bodies that contaminate foliage
for transmission to other larvae.
Once ingested, the occlusion bodies dissolve in the highly alkaline midgut of
phytophagous insects, liberating the occlusion-derived virions for
the next cycle of infection.
|
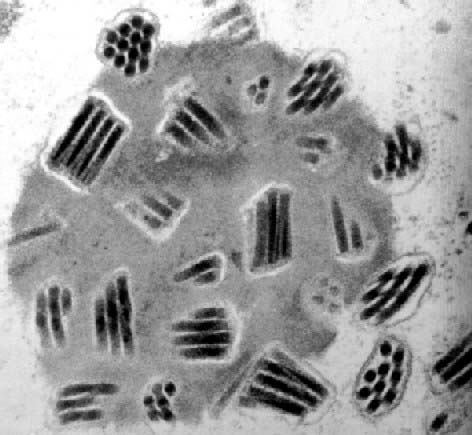
Rod-shaped nucleocapsids are enveloped
to form virions in the
cell nucleus
and occluded by a protein matrix.
|

Nucleopolyhedrovirus
infection of Spodoptera exigua
– a nasty way to die! |
|
|
|
|
|
|
|
|
|
|
|
|
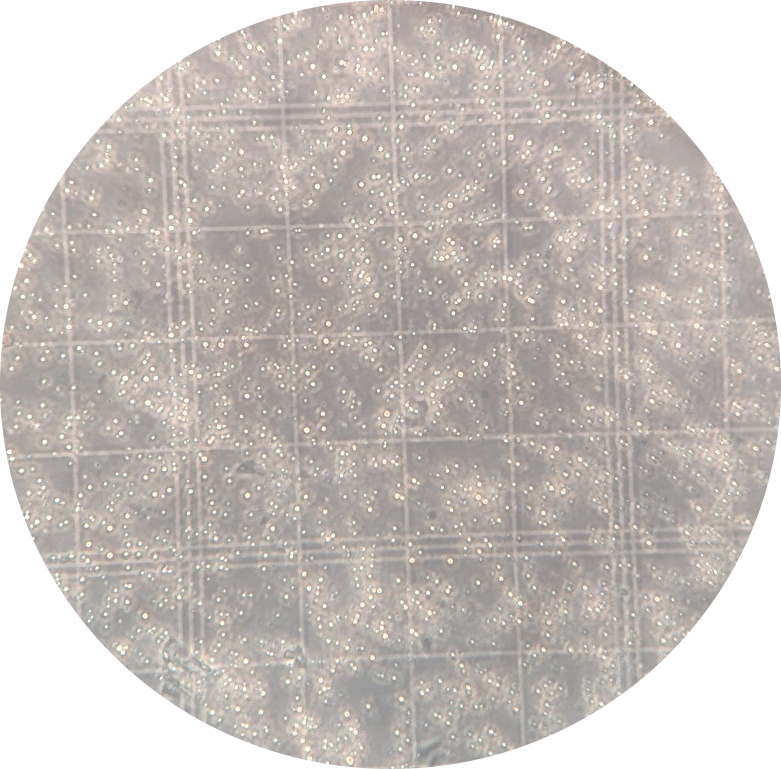
Virus occlusion bodies (seen here as
brilliant dots) can be quantified using a hemocytometer (x400) |
The relatively large size of viral
occlusion bodies (1-3 µm) means that they can be visualized and
quantified by counting under a phase-contrast microscope.
For example, a diluted suspension of OBs placed on the grid of a Neubauer-type hemocytomer can be counted to estimate the number of
OBs present in the original suspension of virus.
Several counts are performed on each suspension and the average
value is calculated to minimize the variation that arises from
dilution effects or handling of small volumes of OB suspension.
Different concentrations of OB suspension can then be prepared and
used to inoculate insects in order to determine the insecticidal
activity of different virus isolates.
The dose of OBs required to infect and kill 50% of the
larvae of a particular developmental stage (instar) is known as the
LD50 (or LC50 in the case of an OB concentration). |
|
|
|
|
|
|
|
|
|
|
|
|

Predatory invertebrates
eat virus-infected prey and subsequently disperse OBs, over
distances of many meters, in their feces (photo R. Lasa).
|
Because most of these viruses only infect a few closely related
species of insects, particularly Lepidoptera, they may interact
passively with other insects to achieve dispersal and/or
transmission to new hosts.
For example, the OBs do not dissolve
in the acid guts of predatory insects.
As a result, predators that consume virus infected hosts may
disperse the OBs during several days and over considerable distances
as they defecate the remains of their infected victim.
Similarly,
parasitoid wasps that have stung an infected insect can act as
vectors introducing the virus to susceptible hosts during subsequent
acts of oviposition.
The ecology of these viruses and the relationship between ecology
and pest control have been reviewed in detail (Williams
2018).
|
|
|
|
|
|
|
|
|
|
|
|
|

A predatory
bug (Nesidiocoris) about to feed on a virus-infected larva.
Bugs can transmit the virus to other larvae because their mouthparts
and body become contaminated with virus particles (photo R. Lasa). |
|
|
|
|
|
|
|
|
|
|
|
|

Virus isolates from insects or from the
soil can be compared by their genetic restriction profile using
enzymes that cut the virus DNA into fragments of different lengths. |
The soil represents a major virus
reservoir in the environment (reviewed in
Williams 2023).
OBs can persist in acid or neutral soils for months or years before
being transported back onto leaf surfaces by rainsplash, air
currents, or by the movement of soil surface dwelling arthropods.
Work by Murillo et al. (2006) indicates that certain genotypes
present in baculovirus populations may be better adapted to survival
in soils than others.
Certain virus genotypes may also be
better adapted to survive in soils of different acidity or
alkalinity.
The principal factors that limit OB persistence in the environment
are solar UV radiation and exposure to alkaline conditions such as
occur in calcium rich soils and on the leaf surfaces of certain
plants (e.g. cotton).
OBs can be isolated from soil samples by mixing the soil with insect
diet and feeding the mixture to susceptible larvae, a technique
developed by Richards & Christian (1999).
Click here to see how we are using
this technique for the study of Spodoptera
nucleopolyhedroviruses in agricultural soils.
|
|
|
|
|
|
|
|
|
|
|
|

Of 186 soil samples collected from maize
fields in Mesoamerica, 35 samples (18.8%) proved positive for SfMNPV
(orange dots). |

Collecting soil samples from a maize
field in Guatemala in 2000 in collaboration with Andy Richards
(CSIRO). |
|
|
|
|
|
|
|
|
|
|
|
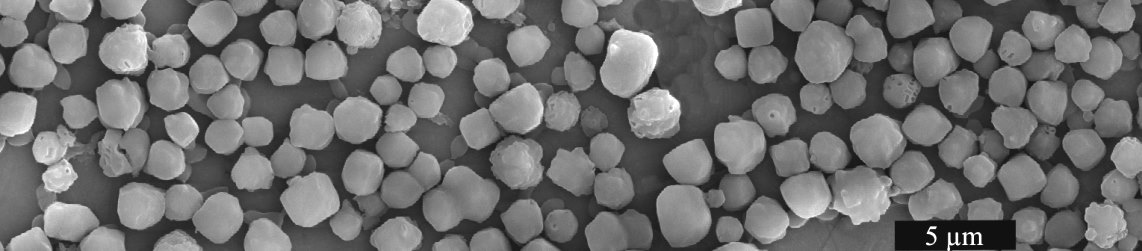
Virus occlusion bodies are highly resistant
and can survive long periods in the environment (scanning
electron microscope image). |
|
|
|
|
|
|
|
|
|
|
|
|
Baculovirus populations are
genetically heterogeneous and individual isolates often comprise a
mixture of different genotypes, including defective variants that
are incapable of achieving transmission or on their own.
The interactions between genotypes can have surprising consequences
for the phenotype of the mixture and the probabilities of
transmission of each of the constituent genotypes.
For example,
we initially demonstrated increases
in the pathogenicity of mixtures of variants containing complete and defective
genotypes (López-Ferber et
al., 2003).
Repeated steps of insect-to-insect transmission of such mixtures
rapidly results in an equilibrium in which the proportion of
defective genotypes is precisely the proportion seen in the wild
population.
When two genotypic variants (or two
distinct nucleopolyhedroviruses) replicate in the same cell, the
nucleocapsids and ODV envelope proteins are shared among the
variants to produce virus particles with a mixed-variant pseudotype
that may differ in its pathogenic characteristics compared to each
of the component variants (Williams
et al., 2022).
This work underlines the importance of genotypic diversity on the
transmissibility and stability of baculovirus
populations (López-Ferber et al.,
2025).
|

Schematic of co-occluded genotypic
variants. Variants (shown in green or blue colours) that replicate
in the same cell become enveloped individually or in groups to form
mixed-variant virions (ODVs). Nucleocapsids have a rod-like
appearance in longitudinal section and are circular in transverse
section. |
|
|
|
|
|
|
|
|
|
|
|
|
|
Publications on baculovirus ecology
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Complete list of publications |
|
Sitemap |
|
HOMEPAGE |
Iridoviruses |
Virus insecticides |
Spinosad |
Mosquitoes blackflies
& ticks |
Predators,
parasitoids, pathogens |
Flies of
agricultural importance |
Students |
Español |
 |










